Master Validation Plan Template
Master Validation Plan Template - You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. The template includes a requirements traceability matrix, a. Items indicated “*” are listed as. Validation master plan template (vmp) describes the overall strategy, approach, and responsibilities for validation of computer systems and software. This article can help you understand the principle of a master validation plan and what is involved in creating one. The following template is suggested for a validation master plan which can be adapted for local use. Download a free template for verification and validation plan in ms word format.
Master Validation Plan Template
This article can help you understand the principle of a master validation plan and what is involved in creating one. The following template is suggested for a validation master plan which can be adapted for local use. You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. A free.
What is Validation Master Plan? (Template, Examples)
This article can help you understand the principle of a master validation plan and what is involved in creating one. You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. The following template is suggested for a validation master plan which can be adapted for local use. Items indicated.
Validation Master Plan Template Printable And Enjoyable Learning
A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. This article can help you understand the principle of a master validation plan and what is involved in creating one. The following template is suggested for a validation master plan which can be adapted for local use. Download.
Master Validation Plan Template
This article can help you understand the principle of a master validation plan and what is involved in creating one. The following template is suggested for a validation master plan which can be adapted for local use. You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. Items indicated.
Validation Master Plan Template Validation Center
A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Download a free template for verification and validation plan in ms word format. You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. This article can help.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
The following template is suggested for a validation master plan which can be adapted for local use. Download a free template for verification and validation plan in ms word format. Items indicated “*” are listed as. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. You might.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Download a free template for verification and validation plan in ms word format. This article can help you understand the principle of a master validation plan and what is involved in creating one. Validation master plan template (vmp).
Validation Master Plan Template Verification And Validation
Validation master plan template (vmp) describes the overall strategy, approach, and responsibilities for validation of computer systems and software. Download a free template for verification and validation plan in ms word format. You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. The template includes a requirements traceability matrix,.
Master Validation Plan Template
You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. Download a free template for verification and validation plan in ms word format. Validation master plan template (vmp) describes the overall strategy, approach, and responsibilities for validation of computer systems and software. This article can help you understand the.
Validation Master Plan. Understand the importance and benefits
Items indicated “*” are listed as. This article can help you understand the principle of a master validation plan and what is involved in creating one. Validation master plan template (vmp) describes the overall strategy, approach, and responsibilities for validation of computer systems and software. Download a free template for verification and validation plan in ms word format. The following.
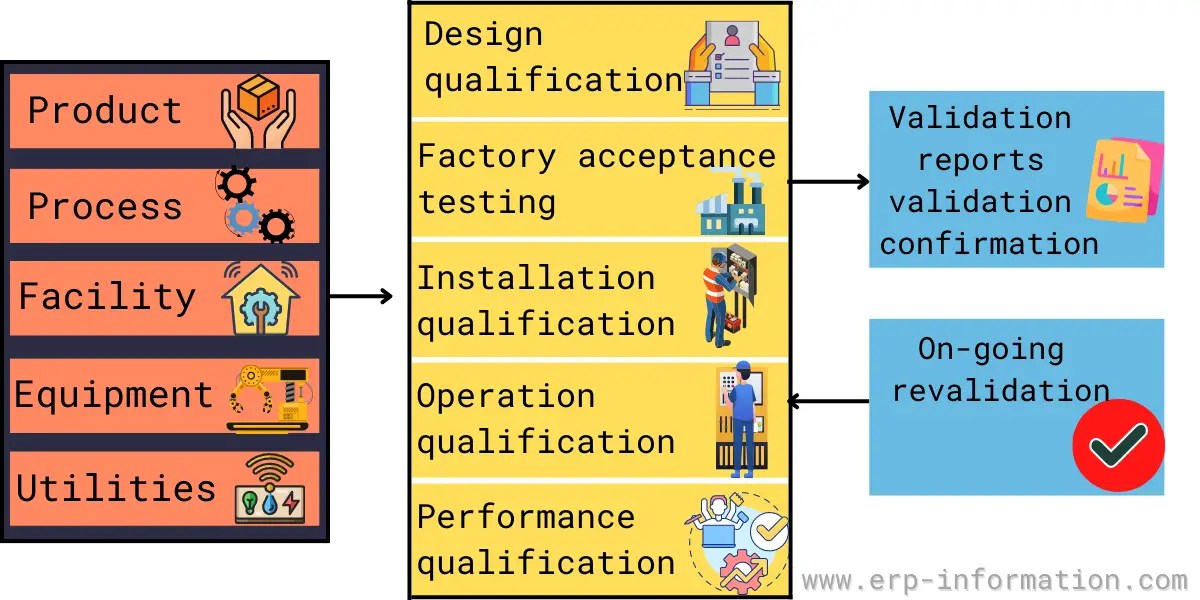
Download a free template for verification and validation plan in ms word format. The following template is suggested for a validation master plan which can be adapted for local use. Items indicated “*” are listed as. This article can help you understand the principle of a master validation plan and what is involved in creating one. The template includes a requirements traceability matrix, a. Validation master plan template (vmp) describes the overall strategy, approach, and responsibilities for validation of computer systems and software. You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation.
Validation Master Plan Template (Vmp) Describes The Overall Strategy, Approach, And Responsibilities For Validation Of Computer Systems And Software.
This article can help you understand the principle of a master validation plan and what is involved in creating one. You might wonder what a master validation plan (mvp) is and how to develop and implement one for your gmp facility. The template includes a requirements traceability matrix, a. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation.
Items Indicated “*” Are Listed As.
Download a free template for verification and validation plan in ms word format. The following template is suggested for a validation master plan which can be adapted for local use.